Overview
The first draft of the Human Genome Project was unveiled recently showing that up to 20% of the 30,000-40,000 genes areinvolved in signaling. There are more than 520 protein kinases and of these 58 have been identified as receptor tyrosine kinases (RTKs) and 32 as cytosolic protein tyrosine kinases.
Protein tyrosine kinases function in a variety of cellular activities, e.g. cell growth, cell cycle progression, differentiation, embryonic development, anti-apoptosis, cell motility and adhesion, gene transcription, angiogenesis, ion channels and the list goes on.
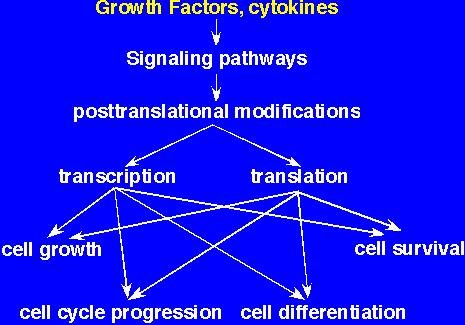
We study one of the RTKs. This is the receptor for CSF-1, which stands for Colony Stimulating Factor-1, also known as M-CSF or Macrophage Colony Stimulating Factor. As the name implies, CSF-1 is a key growth factor for cells of the monocyte-macrophage lineage. The CSF-1 receptor is in the same family as the PDGF, kit and flk/flt receptors. We use the CSF-1 receptor as a model for studying the basic mechanisms underlying the control of cell growth (increase in cell size), proliferation (cell division) and survival (Fig.1). Mutations that upregulate expression of RTKs are frequent in human cancers. Moreover the pathways utilized by RTKs are often constitutively active. Hence both RTKs and their downstream effectors are potential targets for anticancer drug therapy.
Our research program focuses on a basic understanding of the roles of intracellular signaling pathways activated by the CSF-1R in hematopoietic cell culture models as well as primary bone marrow-derived macrophages. We are interested in all pathways that contribute to CSF-1-mediated cell growth and survival.
Tyrosine kinases function in signaling cascades that comprise different classes of molecules: other tyrosine kinases, serine/threonine kinases, lipid kinases, adaptors and scaffolding molecules. We recently found that one of the scaffolding molecules working downstream of the CSF-1R, Gab2, is markedly upregulated when embryonic stem cells are induced to differentiate into neurons. Consistently, primary neural stem cells isolated from the subventricular zone of postnatal mice express high levels of Gab2, which persist in neurons. These findings are very exciting particularly in view of recent reports that Gab2 is a significant risk modifier for Alzhemier's disease. We now wish to understand the role of Gab2 and other members of this family (Gab1 and Gab3) in the central nervous system. Specifically, we would like to know if Gab proteins play a role in maintenance of the neural stem cell pool and neuronal specification, which receptors activate Gab proteins and which signaling pathways are modulated by these molecules.
Fig. 1